Our work on the evolving solid-state chemistry in secondary LiMn2O4 (LMO) particles during lithiation and its impact on the performance of the electrode has been published in a new paper in Nature Communications
Our scientists’ new work on the evolving solid-state chemistry in secondary LiMn2O4 (LMO) particles during lithiation and its impact on the performance of the electrode has been published in a new paper, “Spatial quantification of dynamic inter and intra particle crystallographic heterogeneities within lithium ion electrodes” in Nature Communications.
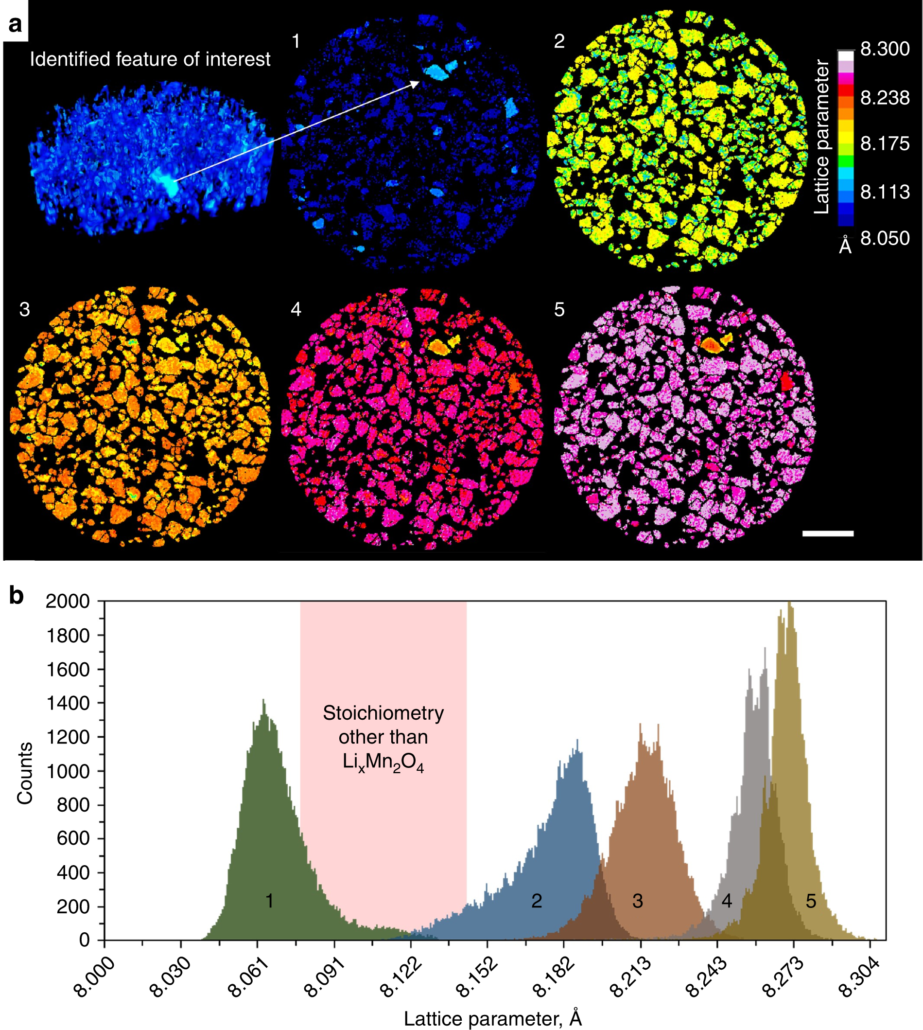
a (Top left) 2 µm resolution multi-slice XRD computed tomogram used to identify a region of interest. (1–5) Sequential 1 µm resolution XRD-CT slices taken during discharge of the Li vs LMO cell, showing the progression of lithiation of the LMO phase. Scale bar is 50 µm. b Histograms composed of the lattice parameter values assigned to each voxel in XRD-CT slices 1–5. The pink region highlights the range of lattice parameter values over which a bi-phasic reaction of LixMn2O4 passes without occupying, i.e., a region that is not characteristic of the spinel LixMn2O4 stoichiometry.
The work was performed with Donal Finegan from the National Renewable Energy Laboratory and in collaboration with a team from the Electrochemical Innovation Lab (EIL) from UCL Chemical Engineering using ESRF’s ID15A beamline.
The performance of lithium ion electrodes is hindered by unfavorable chemical heterogeneities that pre-exist or develop during operation. Time-resolved spatial descriptions are needed to understand the link between such heterogeneities and a cell’s performance. Here, operando high-resolution X-ray diffraction-computed tomography is used to spatially and temporally quantify crystallographic heterogeneities within and between particles throughout both fresh and degraded LixMn2O4 electrodes. This imaging technique facilitates identification of stoichiometric differences between particles and stoichiometric gradients and phase heterogeneities within particles. Through radial quantification of phase fractions, the response of distinct particles to lithiation is found to vary; most particles contain localized regions that transition to rock salt LiMnO2 within the first cycle. Other particles contain monoclinic Li2MnO3 near the surface and almost pure spinel LixMn2O4 near the core. Following 150 cycles, concentrations of LiMnO2and Li2MnO3 significantly increase and widely vary between particles.
Finegan, D.P., Vamvakeros, A., Tan, C. et al. Spatial quantification of dynamic inter and intra particle crystallographic heterogeneities within lithium ion electrodes. Nat Commun 11, 631 (2020).
Read the full article at https://doi.org/10.1038/s41467-020-14467-x
