Battery characterisation
Finden provides analytical insight for the development and improvement of battery technology. We have a number of methods at our disposal to study batteries and battery materials (including formation). We are able to study lab cells, coin cells, pouch cells and conventional cylinder batteries such as AA, AAA and 18650. This includes conventional computed tomography to study mechanical defects but also chemical imaging. Below you will find some published work which demonstrates our capabilities featuring mixture of static and in situ measurements revealing chemistry and deactivation pathways within intact battery devices in different charge states.
Further case studies include:
Li-ion batteries (LiBs), widely used in handheld devices, electric cars, and grid storage systems, have become the linchpin of the energy storage industry. While X-ray imaging methodologies have gained significant traction over the last decade for their diagnostic potential in LiBs, they routinely struggle to provide insight into the chemical makeup within commercial batteries. Here, we performed the first in situ/ operando X-ray diffraction tomography study of a cylindrical Li-ion battery using a commercially available and industrially relevant 18650 NCA Li-ion battery.
 Resolving the chemistry inside commercial cylindrical Li-ion batteries with X-ray diffraction computed tomography (XRD-CT)
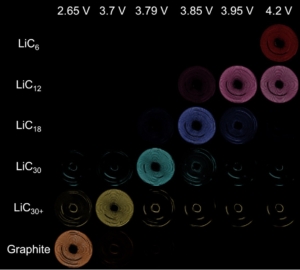
Resolving the chemistry inside commercial cylindrical Li-ion batteries with X-ray diffraction computed tomography (XRD-CT)
Li-ion batteries (LiBs) have dominated the energy storage market finding applications in portable electronic devices, electric vehicles and grid storage. Over the past decade, X-ray imaging techniques have attracted a lot of interest as diagnostic tools for LiBs however conventional methods most often fail to resolve the chemistry inside commercial devices. Synchrotron high-energy X-ray diffraction computed tomography (XRD-CT) was employed to investigate, for the first time, commercial cylindrical Li-ion batteries electrochemically cycled over the two cycling rates of C/2 and C/20.
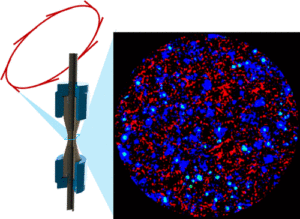
Spatially Resolving Lithiation in Si-graphite Composite Electrodes via in situ High-resolution XRD-CT
Optimizing the chemical and morphological parameters of lithium-ion (Li-ion) electrodes is extremely challenging, due in part to the absence of techniques to construct spatial and temporal descriptions of chemical and morphological heterogeneities. We present the first demonstration of combined high-speed X-ray diffraction (XRD) and XRD computed tomography (XRD-CT) to probe, in 3D, crystallographic heterogeneities within Li-ion electrodes with a spatial resolution of 1 μm.
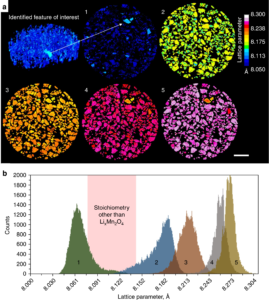
Solid-state chemistry in secondary LiMn2O4 (LMO) particles during lithiation and its impact on the performance of the electrode
The performance of lithium ion electrodes is hindered by unfavorable chemical heterogeneities that pre-exist or develop during operation. Time-resolved spatial descriptions are needed to understand the link between such heterogeneities and a cell’s performance. Here, operando high-resolution X-ray diffraction-computed tomography is used to spatially and temporally quantify crystallographic heterogeneities within and between particles throughout both fresh and degraded LixMn2O4electrodes.
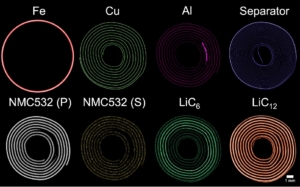
Characterisation of NMC Electodes used in Li-ion Batteries
Environmental considerations and a further electrification of the global automotive fleet and energy grid systems are driving the need for robust electrochemical energy storage devices. While lithium-ion (Li-ion) batteries have emerged over the last few decades as the prime choice for powering portable consumer electronics, they are also being employed for a wider range of applications due to their high energy densities and specific capacities. Since its discovery, lithium cobalt oxide (LCO) has been one of the most commonly used materials in Li-ion electrochemical storage devices and has been employed in numerous applications. High cobalt costs and its inherent toxicity have driven the exploration of other chemistries with reduced Co content, such as lithium nickel manganese cobalt oxide (NMC), where the slightly lower capacity is compensated by excellent power and low self-heating during cycling. However, to meet the demands of modern applications, improvements are required to increase the cycle life, capacity and safety of these materials. We have applied the XRD-CT technique to investigate NMC electrodes cycled at different voltages using a 1 μm X-ray beam.
Read more about our team and publications.